Do Calcium and Rubidium Form an Ionic Compound
Write a formula for the ionic compound that forms between each pair of elements. Start studying Ionic Compounds.
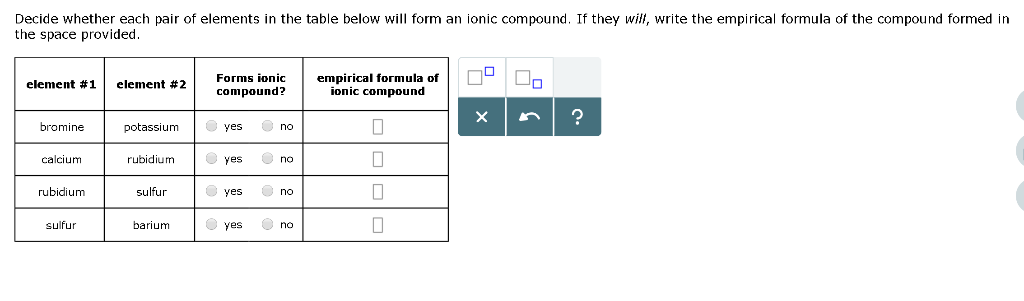
Solved Decide Whether Each Pair Of Elements In The Table Chegg Com
1925 rows Li 2 O 2.
. BaO is the likely formula. Experts are tested by Chegg as specialists in their subject area. Is rubidium chloride an ionic compound.
Learn vocabulary terms and more with flashcards games and other study tools. See answer 1 Best Answer. Calcium ion is Ca2 Ion of.
Therefore the ionic compound that forms between rubidium and bromine is. View the full answer. Rubidium an alkali metal does not form compounds or ionic bonds with calcium an alkaline earth metal.
The subscript for both calcium and oxygen is 1. Kl Determine the chemical formula for the ionic compound that forms between rubidium and sulfur. Element 1 element 2 Forms ionic compound.
Yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium. We review their content and use your feedback to keep the quality high. Empirical formula of ionic compound rubidium oxygen yes O no oxygen calcium O yes O no chlorine calcium O yes O no rubidium magnesium O yes O no This problem has been solved.
Rubidium and bromine d. Li 2 CrO 4. If they will write the empirical formula and name of the compound formed in the spaces provided.
This is because calcium is in group two and so forms ions with a two positive chargeFluorine is in group seven so forms ions with a negative chargeCalcium ions have twice as much charge as fluoride ions. Calcium and oxygen b. A transfer of electrons occurs when fluorine and calcium react to form an ionic compound.
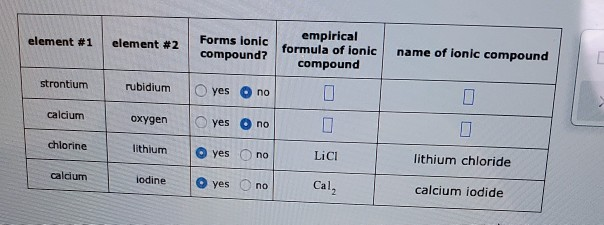
The subscript for both calcium and oxygen is 1. Yes as would be expected from the fact that the electronegativity of chlorine is much. The two each form compounds with several of the same elements eg.
Li 2 Cr 2 O 7. Up to 256 cash back 33. Decide whether each pair of elements in the table below will form an ionic compound.
Li 2 Cr 2 O 7. Empirical formula of ionic compound Forms ionic element 1 element 2 name of ionic compound compound. Zinc and sulfur c.
Rubidium an alkali metal does not form compounds or ionic bonds with calcium an alkaline earth metal. CThe compound formed between rubidium and bromine is an ionic compound because rubidium is a metal and bromine is a non-metal. Ionic bonds are between a metal and a non-metal - Ca and K are both metals.
The charge on both the cation and anion is same and is thus balanced. Rb S Determine the.

Solved Element 1 Element 2 Forms Ionic Compound Empirical Chegg Com
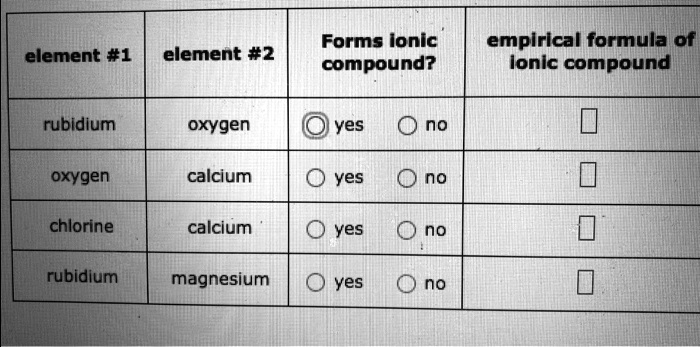
Solved Forms Ionic Compound Empirical Formula Of Ionic Compound Element 1 Element 2 Rubldium Oxygen Yes No Oxygen Calcium Yes No Chlorine Calcium Yes No Rubidium Magnesium Yes No
No comments for "Do Calcium and Rubidium Form an Ionic Compound"
Post a Comment